Research Group Summary
The Berry group focuses on the chemistry of transition metals. They study this by synthesizing, categorizing and classifying, and modeling new metal-metal bonded complexes.
Luke Mangas is a graduate student in the Berry Group. He is studying magnetism in metal-metal complexes. Specifically, he is researching single molecule transistors that have the potential to be smaller than any current transistor. Conventional computers transistors (switches that can be on or off) are what information is stored on and data is processed with. The smaller the transistor, the less energy and better performing the computer is as a whole.
Questions the Berry group asks are
- How does electronic structure impact properties of organometallic complexes?
- Can a single molecule be a magnet?
- How can we explain the magnetic properties of metal complexes?
What Work Looks Like For Luke





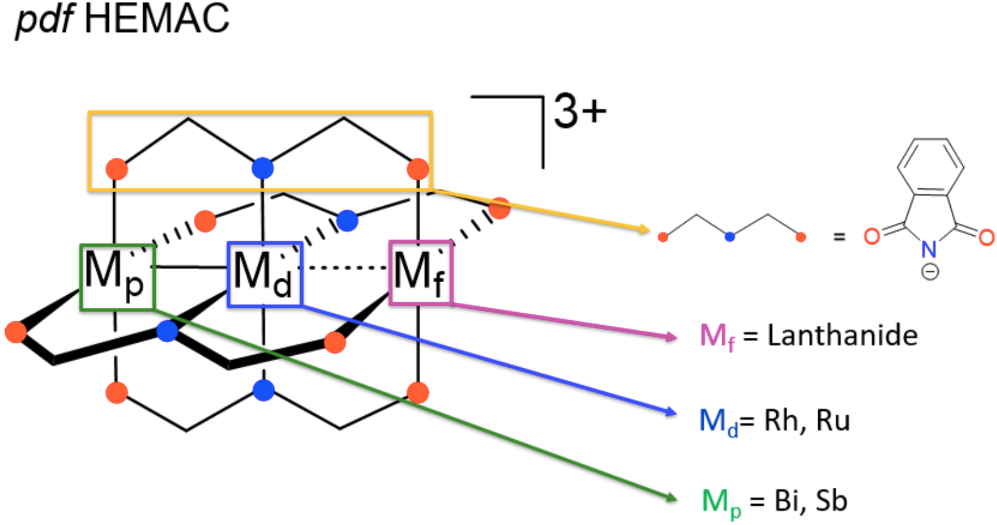
PROCEDURE:
Luke and his colleagues read literature on compounds that are promising. Then they discover a compound that appears to have promising characteristics, they work to synthesize it. They use computational chemistry to create many models of the energies of the different states of the molecule and other properties. They then compare their computational results to the experimentally collected data. They find a model that matches one of the computational models with the experimentally determined values. They finally explore the computational model that worked to find out more about the molecule.
PROGRESS:
Progress right now is focused on increasing the temperature maximum that the molecule can operate as a molecular magnet under. Other experimenters have increased the temperature threshold by 10 degrees, but more increases are necessary in order for these molecular magnets to have practical applications. Regardless of this, more is being understood about these large, symmetrical molecules.
ISSUES:
Computational chemistry modeling struggles to accurately characterize these heavy metal molecules due to relativistic effects.
Currently the molecular magnets are only shown to work at very low temperatures (hundreds of degrees below 0 celsius) This temperature restriction is due to one of the basic natures of atoms and molecules. Molecules are always moving and vibrating. Lower temperatures remove energy from the molecule which allows less vibration and movement. At a low enough temperature/energy, the molecule can not leave the desired state (up or down) without outside intervention. Unfortunately, the current molecules that have been made only work (no ability to change states on their own) at extremely low temperatures.