Phenomena |
Knowledge Claims |
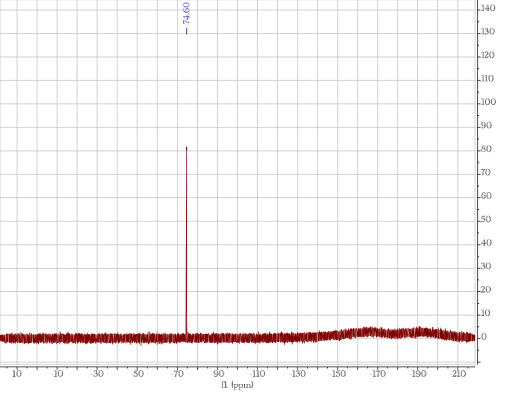
| NMR (hard with paramagnetic items): Nuclear magnetic resonance spectroscopy utilizes the unique character of the nuclei in different atoms (a hydrogen nuclei responds differently than a carbon nuclei) to determine what atoms are in a sample.
F-NMR in CDCl3 |
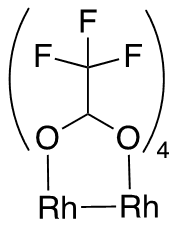
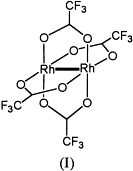
The connectivity of atoms in the molecule is equivalent to one represented by this drawing: |
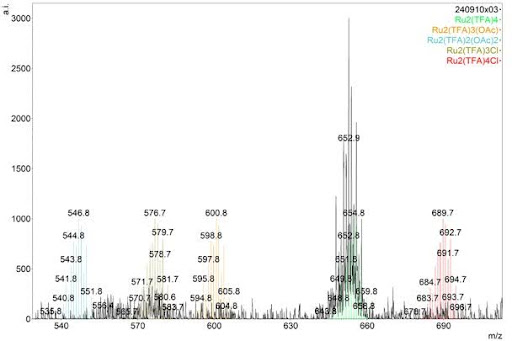
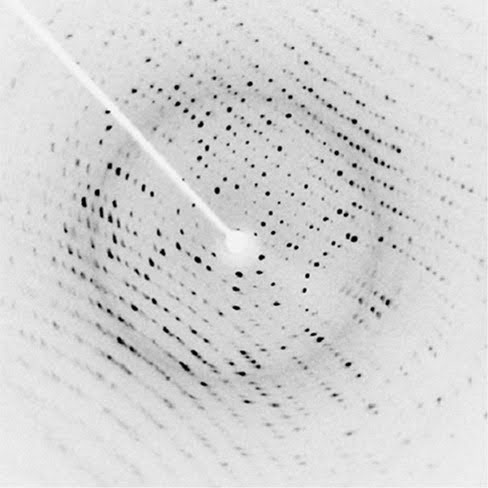
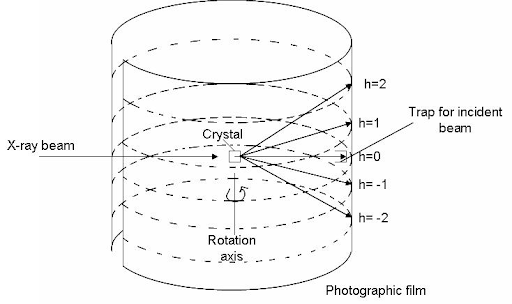
| Mass spec: Stresses molecules to a breaking point. Some bonds in molecules are weaker than others, so those bonds break more often. Because atoms have a known mass, the mass of the broken fragments of molecules can be measured and the makeup of the molecule can be reverse engineered. For example, bismuth has a mass of 209. If one reading of the molecule (unbroken) is 411 and another is 202, the atom broken off is very likely to be bismuth. Crystallography (yeilds most useful data): Supersaturates (packs a ton of molecules of interest into) a solution with the molecule being studied and slowly cools to form a crystal composed of that molecule. X-rays are shot at the crystal and the x-rays are diffracted (their path is influenced by the atoms in the molecule) to show a pattern that is unique for each different molecule.
|
The structure of the molecule is equivalent to one represented by this drawing: |
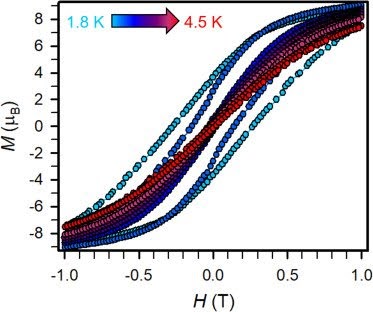
| Hysteresis Graph: X axis is the applied field and Y axis is how magnetized it is.
|
The width of the cycle (space between same colored dots) shows that the magnetization is holding after the removal of the applied field. The higher temperature dots have a narrower cycle width which indicates that the molecule is not holding its magnetization at that temperature as well as at the lower temperature. |