Scientific Models Utilized by Berry Group
Atomic Theory
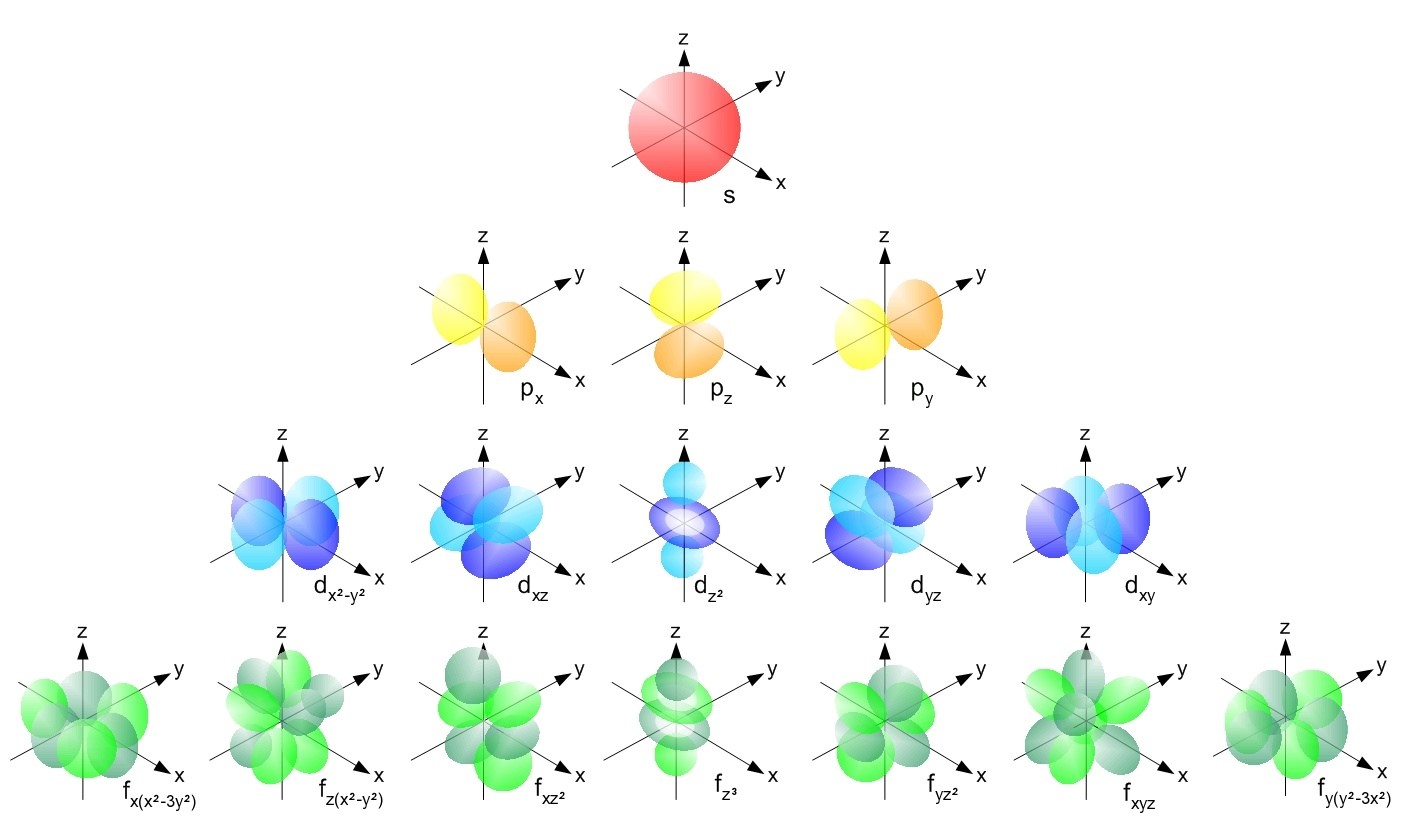
Matter is made of of small particles called atoms. Atoms are composed of a number of protons, neutrons, and electrons. The number of protons in an atom for each element determines the properties of that element. Atoms are best modeled by the wavefunctions of their electrons which are described by quantum mechanics.
Coordination Chemistry
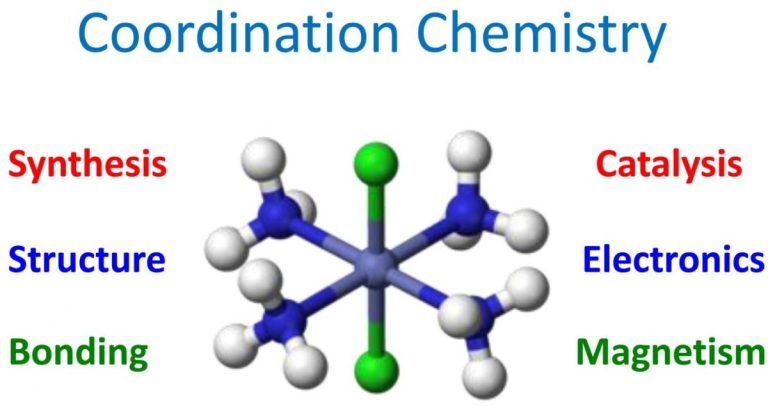
Metal complexes are molecules with a metal center bound to ligands which "donate" electrons to the metal(s). Ligand is a term for a atoms, ions or molecules donating the electrons.
Crystal Field Stabilization Energy
How the splitting of d, p, and f orbitals into different energy levels impacts stability, reactivity, and magnetic properties.
Relativistic Quantum Chemistry
Heavier elements have electrons with velocities which are high enough that a correction needs to be made from quantum mechanics because of special relativity. These corrections are called "relativistic effects", and are difficult to predict from computational models.